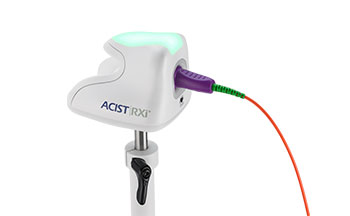
ACIST RXi® System
ACIST RXi® System
for Coronary Physiology
Rapid Exchange FFR System with the Navvus® II
MicroCatheter for Coronary Physiology
ACIST RXi® System
RXi and the ACIST Navvus® II MicroCatheter give you the freedom to quickly and easily assess Fractional Flow Reserve (FFR) using your 0.014″ guidewire of choice along with a simple and intuitive user interface and flexible mounting configurations.

Simple
Plug-and-Play System.
Easy
The touch screen makes it easy to read both aortic and distal pressures as well as FFR and the resting Pd/Pa ratio on one screen.
Adaptable
Offered on a cart or bedrail mounted configuration. It’s flexible to work with your cath lab set-up.
ACIST RXi® Mini™

Flexible
Four unique, adaptable mounting configurations to accommodate your preferred workflow.
Integrated
Seamless with hemodynamic systems for streamlined patient data management.
Small
Minimal cath lab footprint.
ACIST Navvus® II

ACIST RXi® knowledge leadership
Monorail Microcatheter: Dr. Arnold Seto
Assessing complex FFR
RXi offers you the power to simplify assessment of complex coronary artery disease.
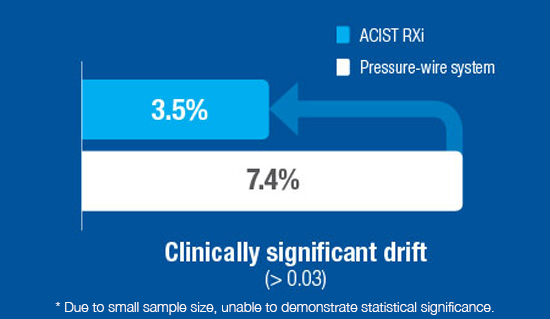
Providing value
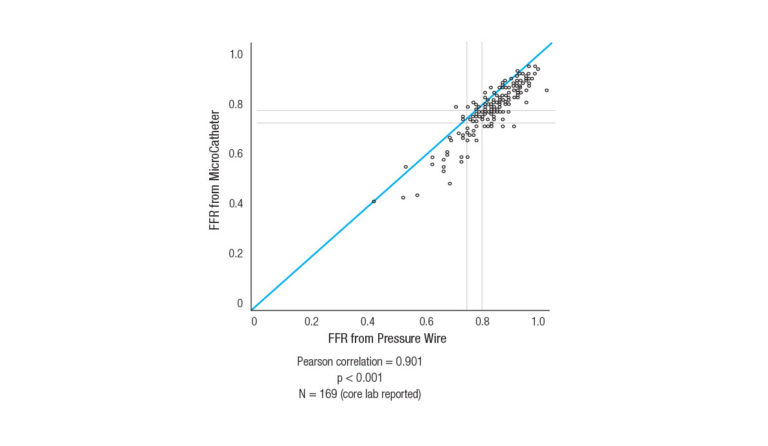
Clinically proven
Reference
1. Fearon WF, Chambers JW, Seto AH, et al. Circ Cardiovasc Interv. December 2017;10(12):e005905.
Rx Only
Prior to use, reference Instructions for Use, inside the product carton (when available) or at < enterwebsite.com > for more detailed information on safe use of the device.
Indications for Use:
The ACIST RXi System and the RXi Mini are indicated for obtaining intravascular pressure measurements for use in the diagnosis and treatment of coronary and peripheral artery disease. The ACIST Navvus® II & Navvus® II MicroCatheter is intended for use with the entire family of ACIST RXi Systems.
Contraindications:
The ACIST Navvus® II Catheters are contraindicated for use in the cerebral vasculature.
Important Safety Info:
The RXi System is to be used only on order of a physician by medical professionals with adequate training and experience in the operation of the RXi System and angiographic procedures and techniques. Additionally, individuals using this device must be alert and
attentive to the operation of the system while it is connected to the patient catheter. Diligence on the part of the user is an essential requirement of overall device safety. The RXi system is not intended for use as a blood pressure monitoring system. Do not use for blood pressure monitoring. RXi Mini is not intended to be used with high frequency surgical equipment.
Prior to use and whenever possible during the procedure, carefully inspect the Navvus® II Catheter for kinks or any other damage. Do not use a kinked or damaged catheter because vessel damage and/or inability to advance or withdraw the catheter may occur. When delivering the Navvus® II Catheter over the guidewire, ensure that the guidewire and the Navvus® II Catheter are freely moving within the vessel wall. Failure to do so may traumatize the vessel. The Navvus® II Catheter is not designed to be torqued. Do not excessively torque the catheter. Never advance or withdraw the Navvus® II Catheter against resistance until the cause of the resistance is determined by fluoroscopy. Movement of the catheter or guidewire against resistance may result in separation of the catheter or guidewire tip, damage to the catheter, or vessel perforation.
This product should not be used in rooms containing magnetic resonance imaging (MRI) equipment.
To avoid inaccurate arterial pressure measurements, the use of guide catheters larger than 8F or guide catheters with side holes are not recommended. The Navvus® II Catheter is not compatible with 4F guide catheters. Do not perform high pressure (> 600 psi) fluid injections while the Navvus® II Catheter tip is inside a guide catheter.
Potential complications that may be encountered during all catheterization procedures include, but are not limited to: vessel dissection or occlusion, perforation, embolus, spasm, local and/or systemic infection, intimal disruption, distal embolization of blood clots and plaque, myocardial infarction, serious arrhythmias, or death.